Introducing the Gram Formula Mass Worksheet Answers, an invaluable resource for students and professionals seeking a comprehensive understanding of gram formula mass calculations. This guide provides a clear and concise overview of the concept, step-by-step instructions for calculating gram formula mass, and a wealth of practice problems to enhance your proficiency.
Throughout this guide, we will explore the applications of gram formula mass calculations in chemistry and delve into extensions of the concept, such as empirical formula determination. By the end of this journey, you will be equipped with the knowledge and skills necessary to confidently tackle any gram formula mass calculation.
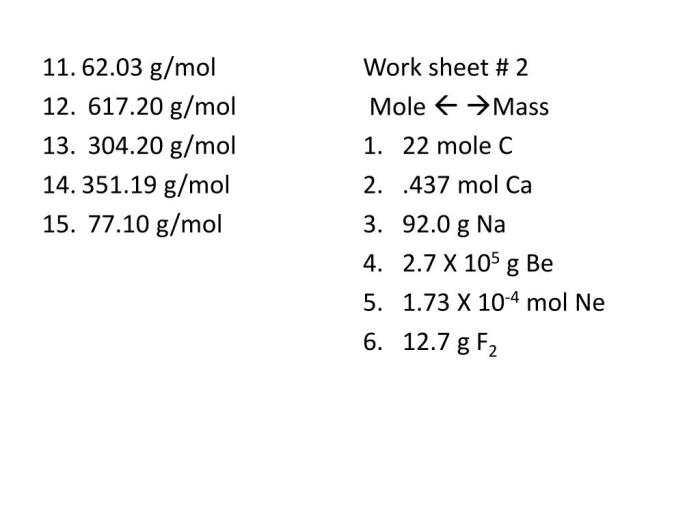
Gram Formula Mass Calculations: Gram Formula Mass Worksheet Answers
Gram formula mass (GFM) adalah massa satu mol suatu senyawa dalam gram. Ini merupakan nilai penting dalam kimia karena memungkinkan kita untuk menentukan jumlah zat dalam suatu sampel.
Untuk menghitung GFM suatu senyawa, kita perlu mengetahui massa atom dari setiap unsur penyusunnya dan jumlah atom setiap unsur dalam rumus empiris senyawa tersebut. Massa atom dapat ditemukan pada tabel periodik.
Worksheet Format and Structure
Tabel berikut menunjukkan struktur worksheet untuk perhitungan GFM:
| Compound Name | Chemical Formula | Molar Mass (g/mol) | Gram Formula Mass (g) |
|---|---|---|---|
| Sodium chloride | NaCl | 58.44 | 58.44 |
| Water | H2O | 18.02 | 18.02 |
| Carbon dioxide | CO2 | 44.01 | 44.01 |
Sample Calculations, Gram formula mass worksheet answers
Berikut adalah beberapa contoh perhitungan GFM:
- Sodium chloride (NaCl)
- Massa atom Na = 22,99 g/mol
- Massa atom Cl = 35,45 g/mol
- GFM NaCl = (22,99 g/mol + 35,45 g/mol) = 58,44 g
- Water (H2O)
- Massa atom H = 1,01 g/mol
- Massa atom O = 16,00 g/mol
- GFM H 2O = (2 x 1,01 g/mol + 16,00 g/mol) = 18,02 g
- Carbon dioxide (CO2)
- Massa atom C = 12,01 g/mol
- Massa atom O = 16,00 g/mol
- GFM CO 2= (12,01 g/mol + 2 x 16,00 g/mol) = 44,01 g
Practice Problems
Berikut adalah beberapa masalah latihan untuk perhitungan GFM:
- Hitung GFM dari kalsium oksida (CaO).
- Hitung GFM dari asam sulfat (H2SO 4).
- Hitung GFM dari metana (CH 4).
Applications and Extensions
Perhitungan GFM memiliki berbagai aplikasi dalam kimia, termasuk:
- Menentukan jumlah zat dalam suatu sampel
- Mengkonversi antara massa dan jumlah mol
- Menentukan komposisi persentase senyawa
Selain itu, konsep GFM dapat diperluas ke konsep lain, seperti:
- Penentuan rumus empiris
- Stoikiometri reaksi kimia
- Kesetimbangan kimia
FAQ Compilation
What is the purpose of a gram formula mass worksheet?
A gram formula mass worksheet provides a structured framework for practicing and mastering the calculation of gram formula mass, an essential concept in chemistry.
How do I calculate the gram formula mass of a compound?
To calculate the gram formula mass, multiply the molar mass of the compound by its chemical formula.
What are the applications of gram formula mass calculations in chemistry?
Gram formula mass calculations are widely used in chemistry to determine the mass of reactants and products in chemical reactions, calculate solution concentrations, and analyze experimental data.
