Experiment 9 a volumetric analysis pre lab – Experiment 9: A Volumetric Analysis Pre-Lab introduces the fundamental concepts and techniques of volumetric analysis, a cornerstone of chemical experimentation. This pre-lab guide provides a comprehensive overview of the experiment, guiding students through the essential steps of preparing for a successful laboratory experience.
Volumetric analysis, a highly precise analytical technique, plays a crucial role in chemistry, enabling the determination of the concentration of unknown solutions through careful measurements and calculations. This experiment provides hands-on experience with the principles and applications of volumetric analysis, equipping students with a solid foundation for future chemical investigations.
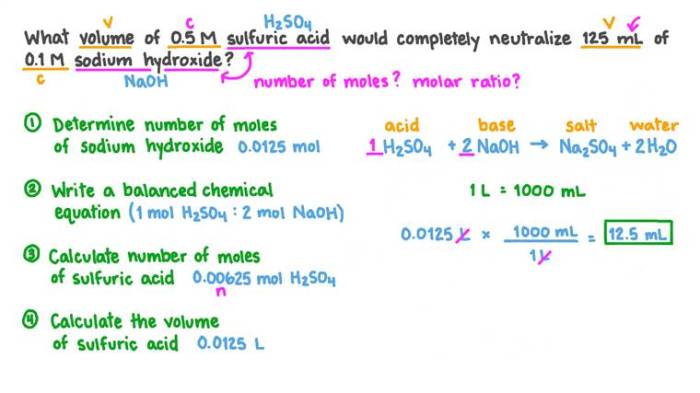
Introduction: Experiment 9 A Volumetric Analysis Pre Lab
The purpose of this experiment is to introduce students to the basic principles of volumetric analysis. Volumetric analysis is a technique used in chemistry to determine the concentration of a solution by reacting it with a solution of known concentration.
Volumetric analysis is a versatile technique that can be used to analyze a wide variety of solutions. It is commonly used in quality control, environmental monitoring, and research.
Materials and Equipment
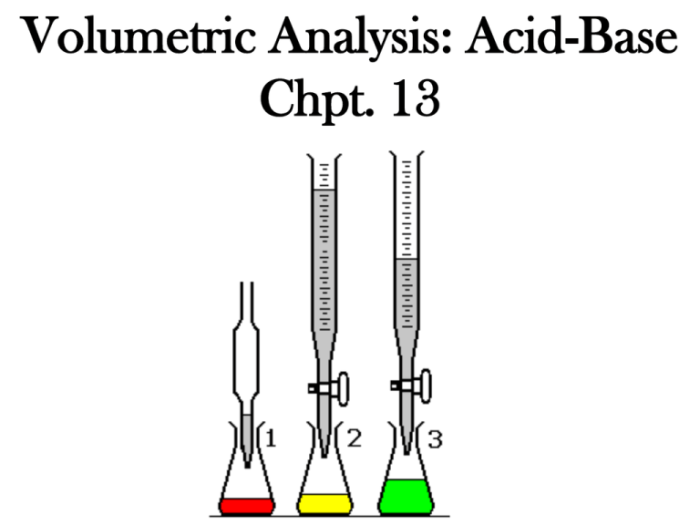
The following materials and equipment are required for this experiment:
- Burette
- Pipette
- Volumetric flask
- Graduated cylinder
- Erlenmeyer flask
- Phenolphthalein indicator
- Sodium hydroxide solution
- Hydrochloric acid solution
The burette is used to deliver a known volume of sodium hydroxide solution to the Erlenmeyer flask. The pipette is used to add a few drops of phenolphthalein indicator to the Erlenmeyer flask. The volumetric flask is used to prepare a known volume of hydrochloric acid solution.
The graduated cylinder is used to measure the volume of hydrochloric acid solution that is added to the Erlenmeyer flask.
Procedure
- Fill the burette with sodium hydroxide solution.
- Pipette a few drops of phenolphthalein indicator into the Erlenmeyer flask.
- Fill the volumetric flask with hydrochloric acid solution.
- Add hydrochloric acid solution to the Erlenmeyer flask until the solution turns pink.
- Record the volume of hydrochloric acid solution that was added.
Data Analysis

The concentration of the sodium hydroxide solution can be calculated using the following formula:
“`M1V1 = M2V2“`where:
- M1 is the concentration of the sodium hydroxide solution
- V1 is the volume of the sodium hydroxide solution
- M2 is the concentration of the hydrochloric acid solution
- V2 is the volume of the hydrochloric acid solution
In this experiment, the concentration of the hydrochloric acid solution is known. Therefore, the concentration of the sodium hydroxide solution can be calculated by rearranging the formula as follows:
“`M1 = M2V2/V1“`
Discussion
The results of this experiment can be used to determine the concentration of a sodium hydroxide solution. This information can be used to control the concentration of sodium hydroxide solutions in a variety of applications.
For example, sodium hydroxide solutions are used in the manufacture of soap, paper, and textiles. The concentration of the sodium hydroxide solution must be carefully controlled in order to produce products that meet specifications.
Questions Often Asked
What is the purpose of Experiment 9: Volumetric Analysis Pre-Lab?
Experiment 9: Volumetric Analysis Pre-Lab provides students with a comprehensive overview of the principles and techniques of volumetric analysis, preparing them for a successful laboratory experience.
What is the importance of volumetric analysis in chemistry?
Volumetric analysis is a highly precise analytical technique that enables chemists to determine the concentration of unknown solutions through careful measurements and calculations. It is widely used in various fields of chemistry, including analytical, environmental, and pharmaceutical chemistry.
What materials and equipment are required for Experiment 9?
The materials and equipment required for Experiment 9 include burettes, pipettes, volumetric flasks, a balance, and various chemicals. The pre-lab guide provides a detailed list of all necessary items.
How do I prepare for Experiment 9?
To prepare for Experiment 9, students should carefully read the pre-lab guide, understand the concepts of volumetric analysis, and familiarize themselves with the experimental procedure. They should also review safety protocols and ensure they have the necessary personal protective equipment.